Timelines for PIP and PSP process. PSP review slide provided from the FDA. | Download Scientific Diagram

Timelines for PIP and PSP process. PSP review slide provided from the FDA. | Download Scientific Diagram
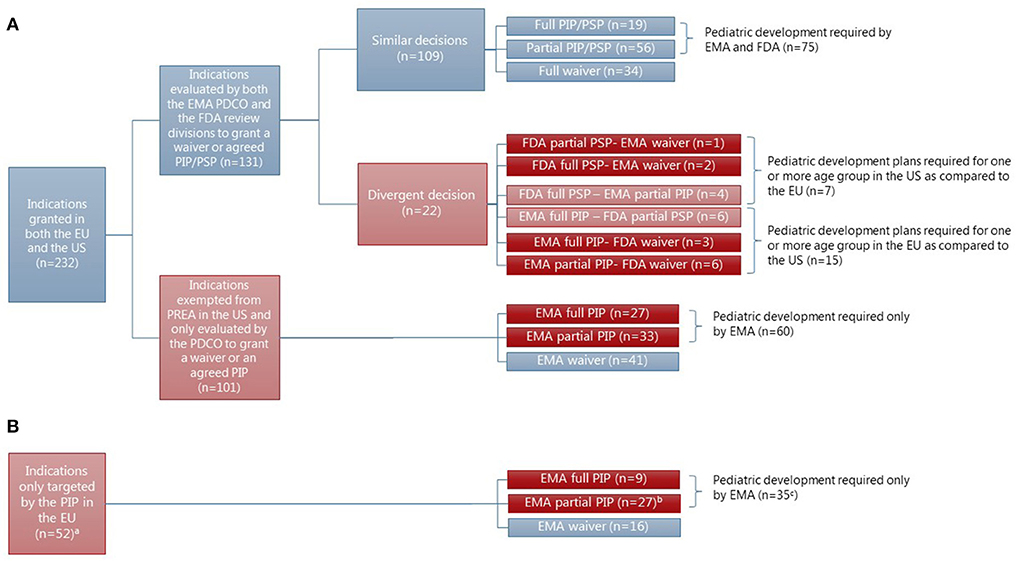
Frontiers | Mandatory requirements for pediatric drug development in the EU and the US for novel drugs—A comparative study

FDA Advisory Meeting Clinical Pharmacology Review Utilizes a Quantitative Systems Pharmacology (QSP) Model: A Watershed Moment? – topic of research paper in Clinical medicine. Download scholarly article PDF and read for free
FDA / EMA Common Commentary on Submitting an initial Pediatric Study Plan (iPSP) and Paediatric Investigation Plan (PIP) for the

Timelines for PIP and PSP process. PSP review slide provided from the FDA. | Download Scientific Diagram
FDA / EMA Common Commentary on Submitting an initial Pediatric Study Plan (iPSP) and Paediatric Investigation Plan (PIP) for the

![PDF] Pediatric drug development in Japan: Current issues and perspectives | Semantic Scholar PDF] Pediatric drug development in Japan: Current issues and perspectives | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/f345b6914fe7338feb66ba7ebcb4f0498f088d32/5-Table2-1.png)